this post was submitted on 09 Aug 2023
871 points (98.4% liked)
linuxmemes
20880 readers
5 users here now
I use Arch btw
Sister communities:
- LemmyMemes: Memes
- LemmyShitpost: Anything and everything goes.
- RISA: Star Trek memes and shitposts
Community rules
- Follow the site-wide rules and code of conduct
- Be civil
- Post Linux-related content
- No recent reposts
Please report posts and comments that break these rules!
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
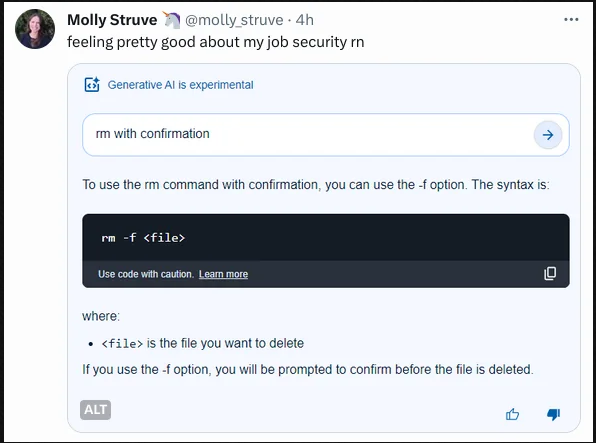
Is this answer correct? I can't judge, as I have no idea how aspirin active compound is synthetized.
Answer from gtp-4:
it's essentially correct, but also it sounds like it was lifted entirely from students lab manual (either chemistry or pharmacy)