this post was submitted on 09 Aug 2023
871 points (98.4% liked)
linuxmemes
20880 readers
5 users here now
I use Arch btw
Sister communities:
- LemmyMemes: Memes
- LemmyShitpost: Anything and everything goes.
- RISA: Star Trek memes and shitposts
Community rules
- Follow the site-wide rules and code of conduct
- Be civil
- Post Linux-related content
- No recent reposts
Please report posts and comments that break these rules!
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
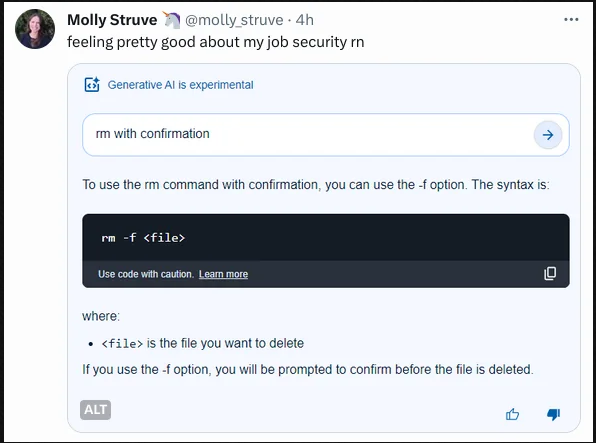
while i get that at some point chatgpt could have been mildly good at bolierplate programming, it's much worse at chemistry. just ask it how to make aspirin
I'm assuming they've blocked out chemistry from the training data. It's crazy how easy it is to make many things from common chemicals, the liability would be insane.
liability.... LOL
No they didn't, gpt-3 was just spectacularly wrong
Is this answer correct? I can't judge, as I have no idea how aspirin active compound is synthetized.
Answer from gtp-4:
it's essentially correct, but also it sounds like it was lifted entirely from students lab manual (either chemistry or pharmacy)