By Alexander Tin
A panel of federal advisers voted Wednesday to back a drugmaker's request to sell a kind of birth control pills over-the-counter, clearing the way for the Food and Drug Administration to approve the first sale of oral contraception on U.S. store shelves without a prescription later this year.
The unanimous vote follows a two-day joint meeting of two FDA committees to weigh a submission by the Perrigo subsidiary HRA Pharma, for their proposed Opill brand product.
Opill is made up of norgestrel, a kind of "progestin-only" birth control pill that was first approved as safe and effective to be prescribed by doctors in the 1970s. This is different from birth control pills that are largely prescribed today, which are newer "combined" formulations that also use estrogen.
The committees had been asked to discuss and vote over whether there was "adequate information to conclude" that the benefits of making norgestrel tablets available over-the-counter outweigh the risks of consumers taking them without a doctor's supervision.
Ahead of the meeting, Perrigo told investors Monday it was expecting the FDA could decide on approval of over-the-counter Opill within the next three months. If approved, Opill could be rolled out as early as the end of this year, a company executive said.
"FDA's approval of Opill for over-the-counter use would address a key unmet need for contraceptive access, be a groundbreaking expansion for women's health nationwide and a step forward toward ensuring people can have improved access to contraception without unnecessary barriers," said Perrigo's Frederique Welgryn in a statement following the vote.
Birth Control Pills
This illustration provided by Perrigo in May 2023 depicts proposed packaging for the company's birth control medication Opill. Perrigo via AP
While the panel's vote is not binding, the FDA says the expert input plays a key role in helping agency reviewers sort through tricky questions posed by drug company submissions. Advisers had wrestled with a range of concerns voiced by the FDA's reviewers with Perrigo's request, including around whether a key study might need to be re-run.
Several participants in a study designed to simulate over-the-counter use of Perrigo's drug reported taking dozens or even hundreds more pills than they were dispensed to take.
"This finding of improbable dosing in this study is really quite extraordinary. This is not something we see in a typical actual use study. And the results have to be incredibly extreme to show up in this kind of a study," the FDA's Theresa Michelle told the panel.
Almost a third of study participants reported taking far more tablets than they were supposed to receive, a result that the FDA worried cast doubt over the validity of all of the results from the study by Perrigo's contractor.
"You really have to wonder about what happened with the other two-thirds if they also over reported, but just not to the extent where we could pick up on it," said Michelle.
But FDA officials also acknowledged the stakes riding on their looming decision, especially as women face growing hurdles in trying to access contraceptives or abortion care.
"The public hearing yesterday was filled with courageous, compelling stories testifying to these challenges," the FDA's Pamela Horn said.
The advisory panel members were skeptical that keeping norgestrel in a prescription-only status, for which it has already been long-approved, would be any better for patients.
"I think all of us, that have had experience taking care of adolescents and adult females, realize that it is very difficult for people to long term take even birth control pills, when they see a provider, the same time every day," University of Texas Medical Branch's Dr. Abbey Berenson, a member of the committee, said.
Benefits and risks of norgestrel
While birth control pills are widely prescribed in the U.S., nearly all American patients take a newer "combined" version that includes an additional estrogen hormone.
Only a small number who need to avoid estrogen take progestin-only pills, which the FDA says must be taken on a stricter regimen to be effective.
"The progestin-only pill is less forgiving, for the reasons that were discussed: it's once daily, and at the same time, allowing for a three-hour window," the FDA's Christine Nguyen said.
Americans also weigh more on average than they did when norgestrel was first approved in the 1970s, a factor which the FDA says research suggests could further diminish norgestrel's efficacy.
More than a hundred countries already allow sale of birth control pills without a prescription, a disparity the FDA acknowledges makes it harder for many Americans to access birth control.
However, the FDA cautioned the panel that most of those countries had widened access to the pills within required guardrails unavailable to U.S. regulators. Pharmacists overseas still screen for issues that could make it unsafe or ineffective for patients to take it, like around potential drug interactions.
"There is no third 'behind the counter' class of drugs in the U.S. like there is in other countries. Drugs in the U.S. are either non-prescription or prescription," the FDA's Pamela Horn said.
 told us, that the cages aren't as fancy under Trump
told us, that the cages aren't as fancy under Trump
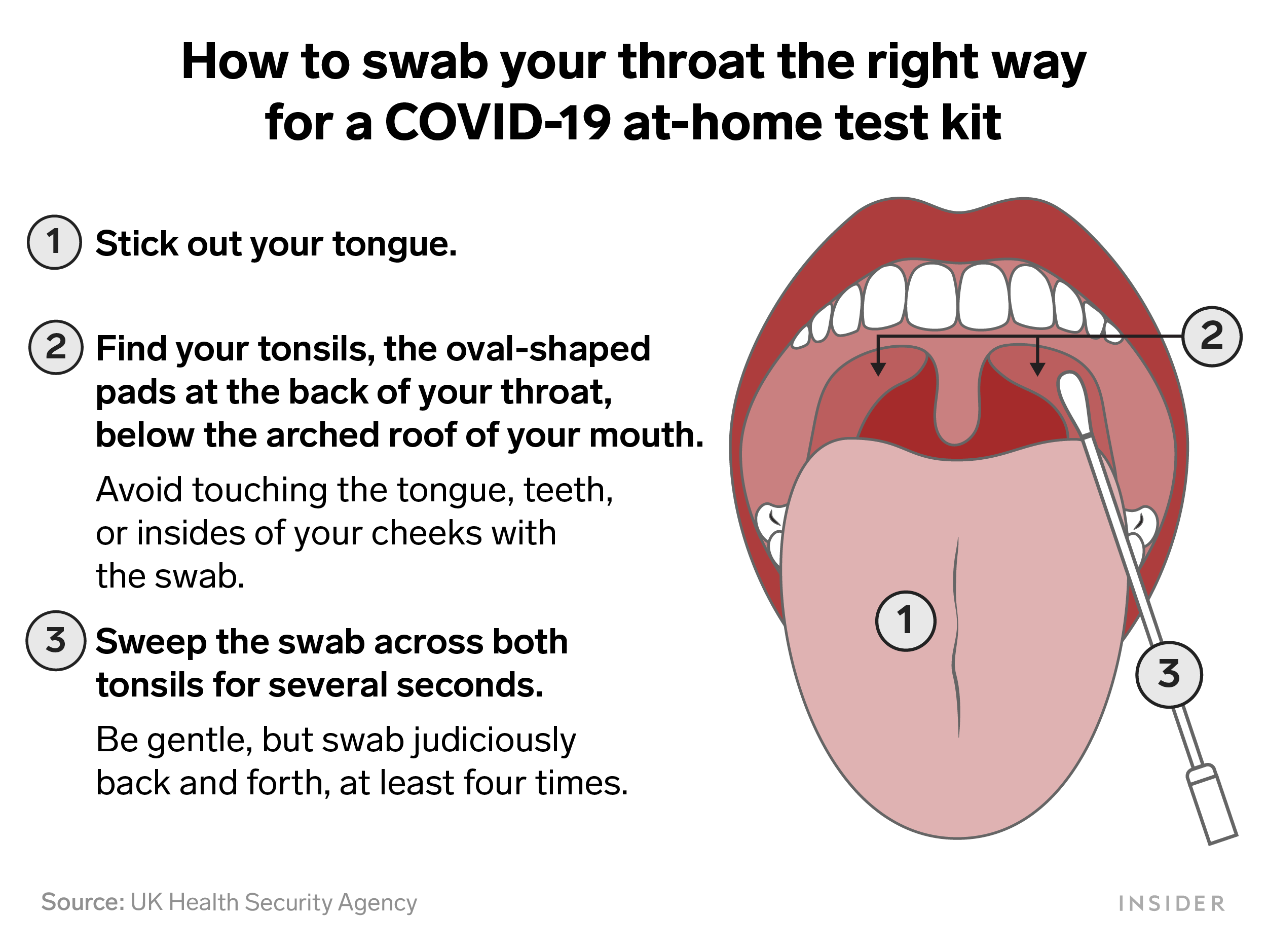
 guiding us to the real viable candidate
guiding us to the real viable candidate

I'm as critical of Brandon as the rest, but he lists his entire platform at joebiden.com smh my head
There's no platform