this post was submitted on 21 Jan 2025
211 points (99.1% liked)
xkcd
10467 readers
5 users here now
A community for a webcomic of romance, sarcasm, math, and language.
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
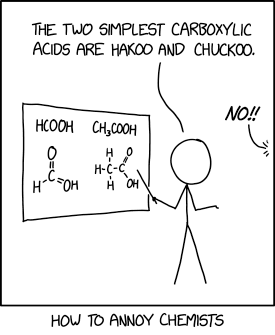
According to that article, someone tried reacting it with just about anything he could find in the lab, and if it weren't for the fact that it's documented in a published paper, one could never be convinced that those experiments are the work of anyone other than a completely deranged scientist. The author of this article claims to have run out of expletives before finishing the second page.
Also stated in this article, and converted for your convenience, reacting FOOF with H~2~S will yield about 1.8 MJ/mol of excess thermal energy.
Most chemists, when you say "fill a reactor vessel at 700 °C with 300 torr of oxygen" and they notice a tank of fluorine gas in the room, will interrupt you and say "no you won't."