this post was submitted on 24 Oct 2024
755 points (97.0% liked)
Science Memes
10827 readers
3396 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
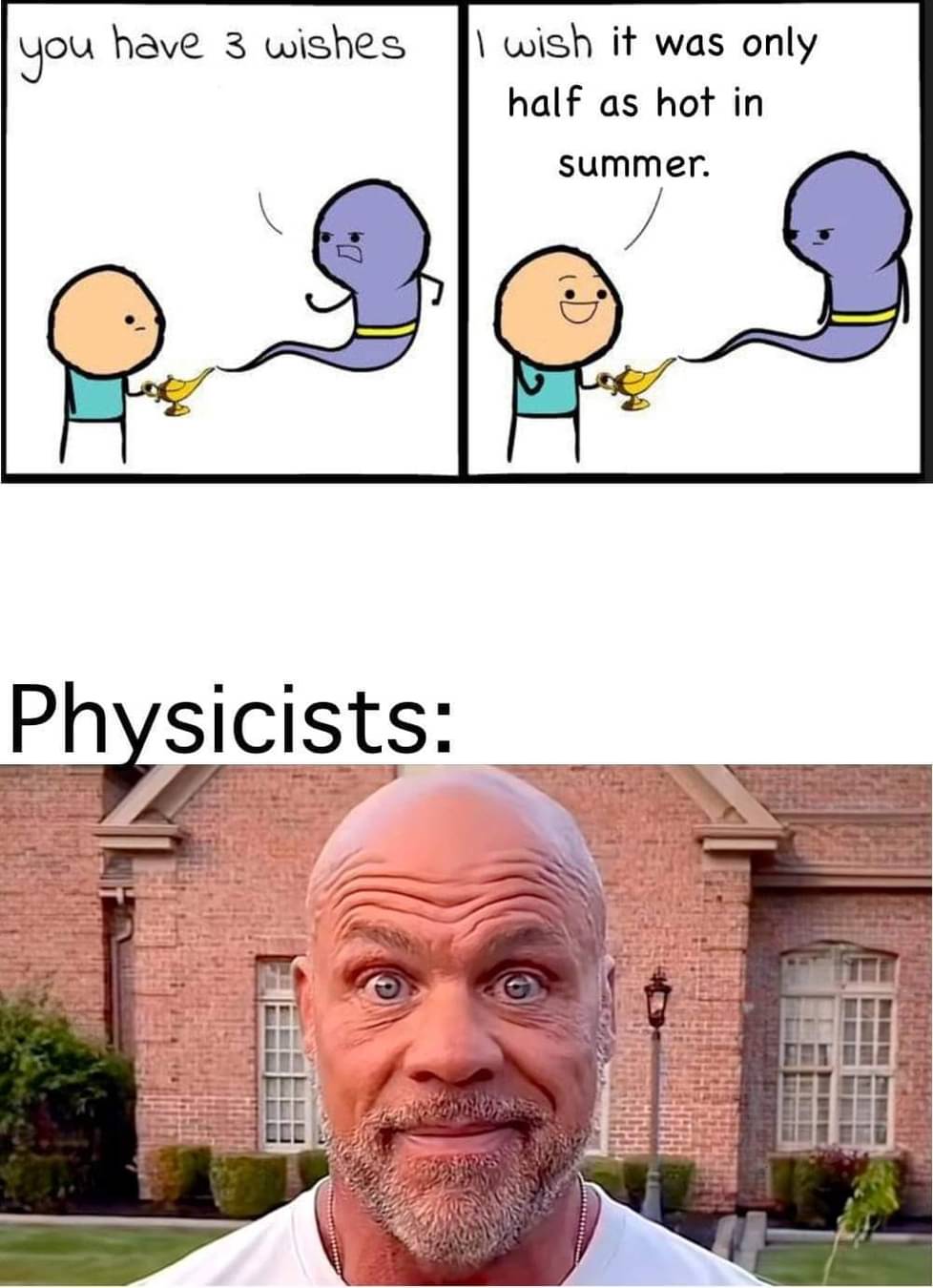
In short, you don't want to use a temperature scale with an arbitrary starting point for doing calculations like this. The freezing point of water is no more or less arbitrary than the freezing point of oxygen or sodium or anything else. It's just one that's somewhat useful for everyday use. When handling calculations for multiplying temperature, you want an absolute scale like Kelvin.
Or Rankine if you're that kind of pervert.
What makes Kelvin absolute, and why is Celsius "wobbly"?
I failed physics in high school
0 K is like when there is 0 heat basically, while celsius isn't. Imagine a unit for distance called "goob" where 0 goobs is 100 m and 1 goob is 115 m. In that case the goob unit would behave differently than a meter when you multiply and divide because 0 of the units don't actually correspond to "nothing" in a physical sense. That's exactly how the Celsius scale is, with zero being placed somewhere arbitrarily, not at a physical zero.
i feel like i need more goob in my life. do you sell rulers?
Bit of an awkward scale for a ruler, but I can sell you one from -6.6666666.... to -6.65 goobs.
absolute scales are still arbitrary. you would probably want to use a scale that measures "perceived heat" which is different than average kinetic energy
Kelvin is just our word for it, but that is the point of “no heat”. It isn’t arbitrary, there is no “negative kelvin” just like you cannot make something colder than absolute zero.
So if you take the difference between “coldest possible temp” and “average summer temp”, then slice it in half, you’re getting temperatures that would kill most life on earth.
Just to nitpick, there are negative kelvins. I don't really understand it, myself, but I know it exists due to the specifics of how temperature is defined. Negative kelvins are actually extremely hot.
just because it has a reason doesn't make it not arbitrary. you can ultimately come up with a reason for all arbitrary decisions
Do you not understand what the word arbitrary means?…
maybe you're the one who doesn't understand the ramifications of its meaning
Is there a way to distinguish between arbitrary and non-arbitrary? Or is literally everything ever arbitrary?
literally every definition ever is arbitrary
Then what's the point of even calling it arbitrary? If it covers everything, then there's no reason for the word.