this post was submitted on 11 Jul 2024
982 points (98.9% liked)
Science Memes
11189 readers
4228 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
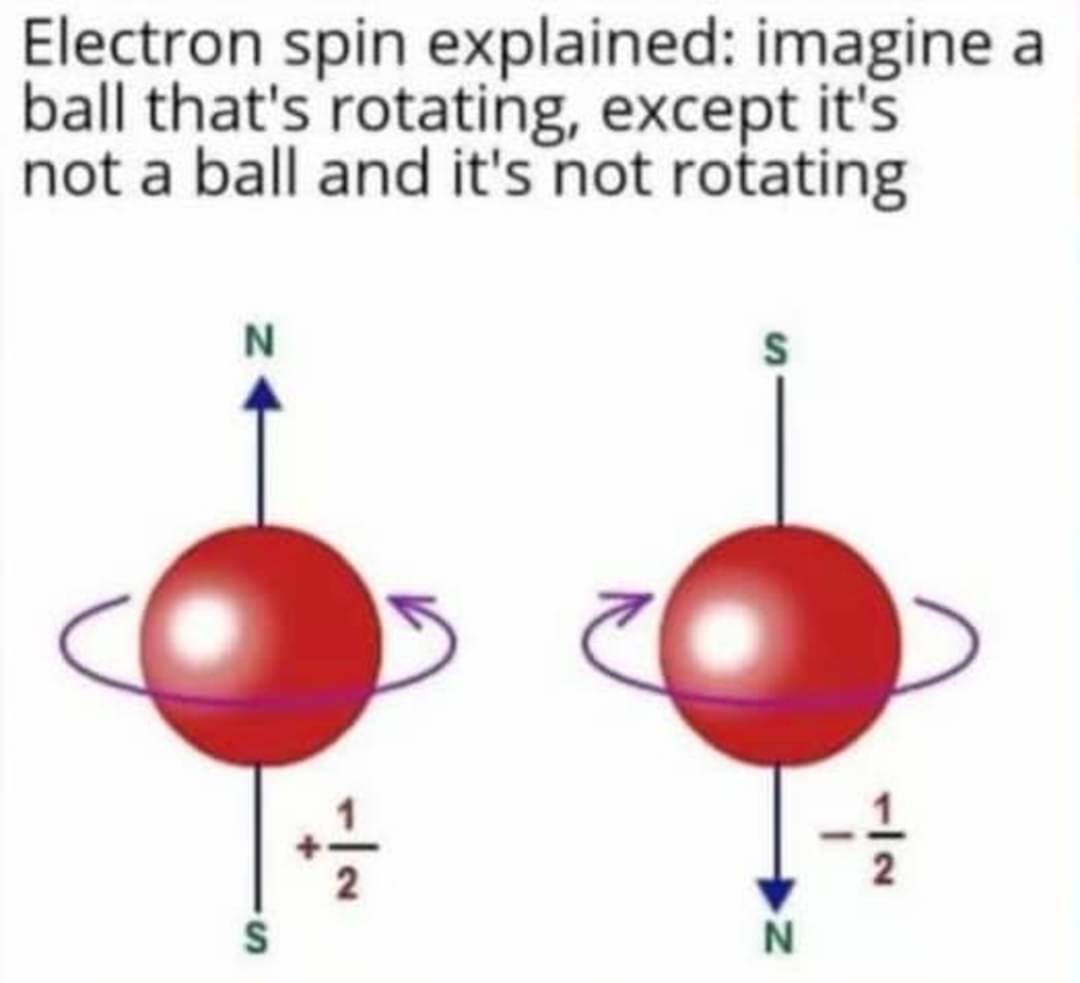
Google "Electron Orbitals". All the spaces there are all the ~~possible~~ highest likely locations for the electrons. Good Introduction to some Quantum Mechanics 👍
No! I will not relive the horrors of that chemistry class again... you can't make me. I am happily an aerospace engineer now where I don't need this chemistry nonsense, or quantum mechanics.
Ah let's see, of the top of my head...
~~1s² 2s² 2d⁶ 3s² 2p¹⁰ ...~~
Edited (iirc now, the d block is in the middle with the transition metals, p block with metallics, Halogens, Noble Gases...):
1s² 2s² 2p⁶ 3s² 2d¹⁰ ...
LMAO
This I was fine with. But that fake make believe redox math? Like are all chemist bad at actual math, so they just came up with their own fake version?
You're a bad person...
Except they only look like that if there is an external reference system imposing some structure on the atom! Otherwise all orbitals are basically spherical because they can all just be in a superposition of all possible orbitals and we couldn't tell a difference...
And then suddenly you have two atoms meeting and need to explain why 1+1=0 for their molecular orbits -.-
I don't think so. Orbitals give you the spaces of highest probability! Electrons could be outside as well. And since it is based on probability it is definitely a useful model.
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals
I'll have a look at this later, I remember it being any possible existence of an election, not just highest probabilities, from when I was taught this several weeks ago.
Ah yes. And if two fields are too close, ~~teleportation~~ tunneling can happen.
In the end, reality is just one big probability engine.